Neuralink Receives US FDA Approval for Tests on Humans
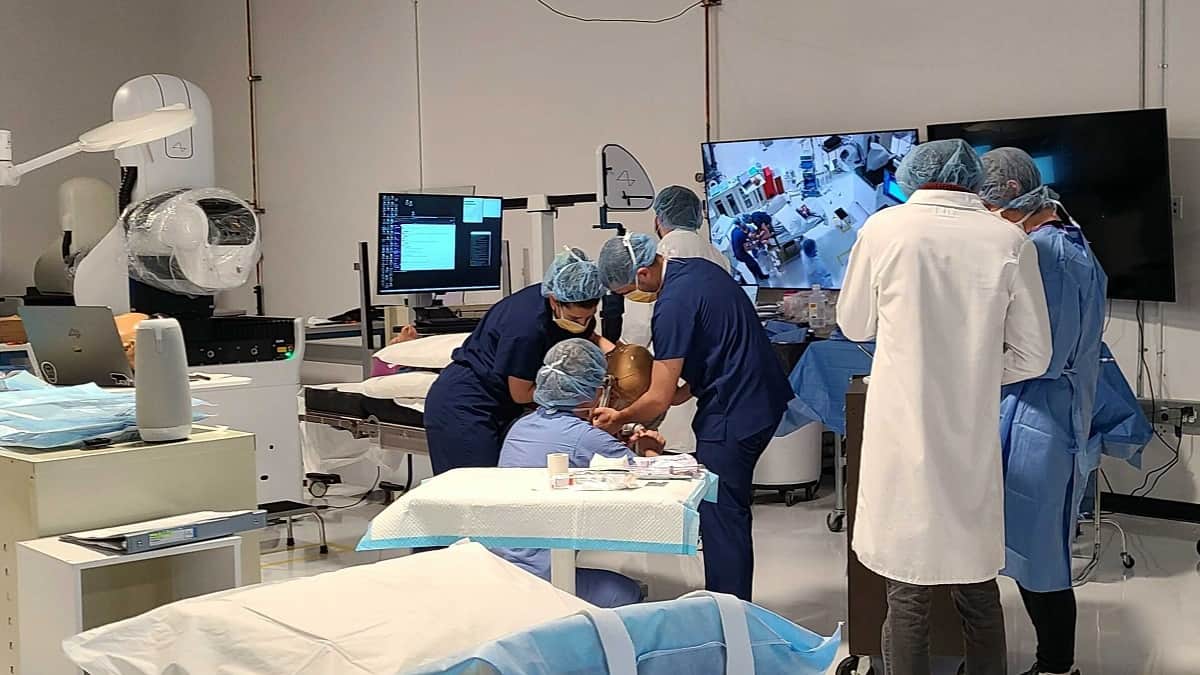
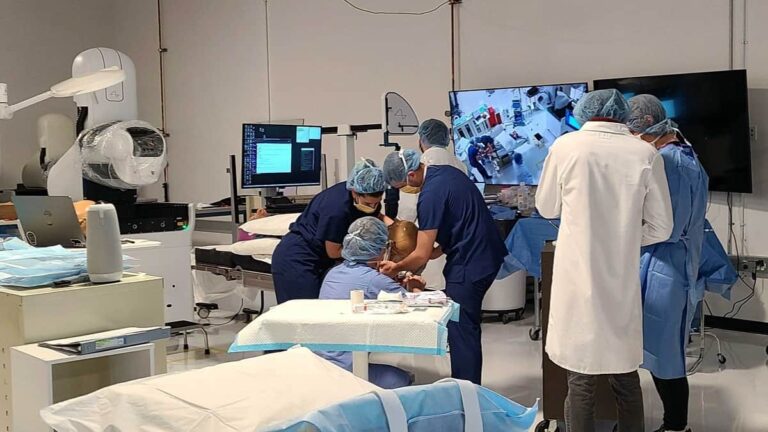
Elon Musk’s brain chip company, Neuralink, has received US Food and Drug Administration (FDA) approval to conduct its first human trials. This is a huge step for the company committed to alleviating human suffering.
Neuralink receives approval for first human trials
Elon Musk’s brain chip company aims to help people with health problems. Specifically, the company wants to help people regain their vision and mobility by connecting their brains to computers. Neuralink announced it has received approval from the FDA to conduct its first human clinical trial. This is a huge breakthrough for the company seeking to limit human suffering.
“We are excited to share that we have received the FDA’s approval to launch our first-in-human clinical study!
This is the result of incredible work by the Neuralink team in close collaboration with the FDA and represents an important first step that will one day allow our technology to help many people.“
Neuralink will not rush to start testing
Neuralink said that receiving the permit does not mean the company will start trials immediately. The company does not plan to start recruiting participants just yet:“Recruitment is not yet open for our clinical trial. We’ll announce more information on this soon!” However, this indicates the developed technology is already so good that it is possible to move on to a new stage of development: human testing. An earlier application by Neuralink in early spring was rejected on safety grounds, the sources, who were not named, said.
Neuralink has already tested chips on monkeys
Neuralink aims to use its chips to treat paralysis and blindness. They can also help people with disabilities use computers and mobile technologies. Previously, the chips have been tested on monkeys. They interpreted the signals generated by the brain and transmitted information to devices via Bluetooth.